Abstract
Introduction: The clinical course of follicular lymphoma (FL) is characterized by recurrent relapses and progressively shorter remissions, with many patients surviving beyond one decade from initial diagnosis. During this time, patients will receive several courses of therapy, which can be complicated by significant toxicities, particularly in elderly patients with multiple comorbidities. While the incidence and outcomes of cardiovascular toxicities observed in subjects with aggressive lymphomas treated with anthracycline based chemotherapy are well known, there is limited information regarding the incidence of cardiovascular events (CVE) in FL patients, many of whom have historically received similar antineoplastic regimens. We conducted a retrospective study to observe the incidence of CVE in FL patients from the time of diagnosis and their effect on patient outcomes.
Patients and Methods: We accessed the Hematologic Malignancies Database of University Hospitals Seidman Cancer Center to identify patients diagnosed with FL between 2002 and 2014. We collected patient data including baseline demographics and risk factors, disease characteristics, treatments prescribed and outcomes, including response, relapse, and incidence of CVE. Multivariate logistic regression modeling was performed to identify risk factors associated with incidence of CVE. Cumulative incidence (with death as competing risk) was used to estimate the incidence of CVE. The Kaplan-Meier method was used to assess overall survival (OS) and progression-free survival (PFS). Two-tailed log-rank test compared OS and PFS curves. All analyses were performed using IBM SPSS Statistics 2015.
Results: Two hundred and twenty-seven FL patients were included for analysis. Median age at diagnosis was 60 years, with 161 patients presenting with advanced stage FL (71%). Approximately two thirds of patients had intermediate or high-risk disease by FLIPI at diagnosis (n= 147, 65%). Decreased ejection fraction at diagnosis was identified in 5 patients (2.2%). Baseline characteristics and initial therapy choice are summarized in Table 1. A total of 143 patients (63%) received chemo-immunotherapy as part of their initial therapy, which included an anthracycline in 99 subjects (44%).
After a median follow up of 66 months, 52 patients (23%) have died. Forty-two patients (18.5%) presented with CVE, with 2 and 5 - year cumulative incidence of 6.4 % (95% CI, 3.9-10.6%) and 13.5% (95% CI, 9.5-19.2%) respectively. Twenty- three patients (10%) developed congestive heart failure while 9 (4%) had an acute coronary syndrome or required coronary artery disease revascularization. Multivariate analysis (Table 2) identified age > 60 years, male gender, prior history of coronary artery disease and anthracycline treatment were independent risk factors for CVE.
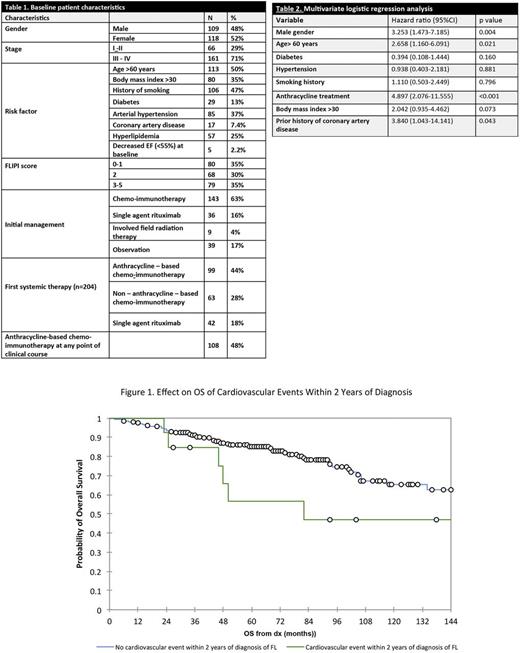
Patients who were prescribed systemic or local therapy after initial diagnosis of FL had a higher cumulative incidence of CVE than patients who were observed initially (5-year cumulative incidence 15.8% vs 9.6 %, respectively) (p=0.043). In addition, subjects who received anthracycline-based therapy at any point in the treatment course presented a higher cumulative incidence of CVE than those never treated with anthracyclines (5-year cumulative incidence 19.1% vs 8.2%, respectively) (p=0.019). The 5-year OS of patients who developed a CVE within 2 years of diagnosis was 56% (95% CI, 27.3-85.6%) versus 85% (95% CI, 80.1-90.3%) in patients who did not develop an early CVE (p=0.048) (Figure 1)
Conclusion: Cardiovascular events in patients diagnosed with FL have a significant effect on their OS. Patients who require treatment are at increased risk of developing cardiovascular events, particularly those treated with anthracycline-based chemo-immunotherapy. Continued investigation is necessary to determine whether novel therapies have the same effect in cardiovascular risk. Interventions to identify, monitor and ameliorate cardiovascular risk factors in patients with FL should be investigated to improve survival.
Malek: Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees; Celgene: Speakers Bureau. Cooper: Novartis: Research Funding. de Lima: Celgene Corporation: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees. Caimi: Abbvie: Equity Ownership; Incyte: Equity Ownership; Celgene: Speakers Bureau; Seattle Genetics: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal